Methodologies for the Analytical Determination of Ferrate(Vi) a Review

Potassium Ferrate (Half dozen) as the Multifunctional Amanuensis in the Treatment of Landfill Leachate
1
Chemiqua Water&Wastewater Visitor, Skawińska 25/1, 31-066 Kraków, Poland
ii
Institute of Chemistry, Academy of Silesia, Szkolna ix, 40-007 Katowice, Poland
3
Department of Water and Wastewater Engineering, Silesian University of Technology, Konarskiego 18, 44-100 Gliwice, Poland
4
Center for Translational Research and Molecular Biological science of Cancer, Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology, Wybrzeże AK 15, 44-101 Gliwice, Poland
v
Section of Analytical Chemistry, Kinesthesia of Natural Sciences, Comenius University, Ilkovicova half dozen, 84215 Bratislava, Slovakia
*
Authors to whom correspondence should exist addressed.
Received: v October 2020 / Revised: 23 Oct 2020 / Accepted: 4 November 2020 / Published: vi November 2020
Abstruse
Possible apply of potassium ferrate (Six) (K2FeO4) for the handling of landfill leachate (pH = 8.9, Chemic Oxygen Demand (COD) 770 mg Oii/L, Total Organic Carbon (TOC) 230 mg/L, Total Nitrogen (Total Due north) 120 mg/L, Total Phosphorus (Total P) 12 mg/L, Total Coli Count (TCC) 6.8 log CFU/mL (Colony-Forming Unit/mL), Most Probable Number (MPN) of fecal enterococci 4.0 log/100 mL, Total Proteolytic Count (TPC) 4.iv log CFU/mL) to remove COD was investigated. Central Composite Pattern (CCD) and Response Surface Methodology (RSM) were applied for modelling and optimizing the purification process. Conformity of experimental and predicted information (R 2 = 0.8477, R adj 2 = 0.7462) were verified using Analysis of Variance (ANOVA). Application of ThouiiFeO4 using CCD/RSM allowed to decrease COD, TOC, Full North, Total P, TCC, MPN of fecal enterococci and TPC by 76.2%, 82.half dozen%, 68.iii%, 91.6%, 99.0%, 95.8% and 99.3%, respectively, by using K2FeO4 0.390 1000/L, at pH = 2.3 within 25 min. Application of equivalent amount of iron (as FeSO4 × 7HiiO and FeCl3 × 6H2O) under the aforementioned conditions allowed to diminish COD, TOC, Full N, Total P, TCC, MPN of fecal enterococci and TPC merely by 38.1%, 37.0%, 20.eight%, 95.8%, 94.4%, 58.ii%, xc.8% and 41.6%, 45.seven%, 29.ii%, 95.8%, 92.1%, 58.2%, ninety.0%, respectively. Thus, GrandtwoFeOfour could be applied as an environmentally friendly reagent for landfill leachate treatment.
i. Introduction
Several aspects of human agricultural and industrial activeness are related to adverse changes in the quality of water resources worldwide. Undoubtedly, such activeness has a direct impact on the waste production. In fact, some waste matter undergoes various disposal processes while other is deposited in municipal landfills. As a result of the municipal waste landfills exploitation, leachate is generated. In case of insufficient protection, it may get into the soil or groundwater and, due to its physicochemical and microbiological limerick, this may significantly contribute to the groundwater contamination. The amount of leachate and their characteristics depend on a number of factors, including: type of waste, caste of fragmentation, compaction and storage method, landform, corporeality of precipitation, method of sealing the bottom of the landfill, type of vegetation covering the landfill, soil weather, etc. [1]. It is widely known that the values of parameters (e.yard., COD, TOC etc.) for the landfill leachate were higher in the dry season than in the rainy flavour for the fresh leachate samples. Recent enquiry points to a higher content of heavy metals in the suspensions. Moreover, in that location were no significant seasonal changes in the concentration of heavy metal ions in suspended solids and sediment samples [2].
However, depending on the age of the landfill (young < 5 years; medium v–x years; erstwhile > 10 years), the physicochemical composition of the leachate may vary [3,iv]. According to the available data [iv,five,6,7,8,9,10,11,12], the pH-value of the leachate varies in the range of 4–7.half-dozen, 6.9–9 and 8.1–9.5 for the leachate from immature, medium and erstwhile landfills, respectively. In the case of COD and TOC, a decrease in the values of these indicators was observed co-ordinate to the age of the landfills, and for COD they amounted to approx. ane.87–84.30, 0.56–9.50 and 0.ten–3.46 g O2/L [4,13,14,15,xvi], and for TOC approx. 1.60–13.61 m/L, 0.19–2.05 g/L and 0.04–1.90 g/L [sixteen,17,18,nineteen,20,21,22,23]. Similar dependencies were observed in the case of full nitrogen (approx. 1.75–4.37, 0.35–iii.00 and 0.42–ii.64 k/L) and full phosphorus (approx. ii–655, 3–18 and 1–seven mg/L) [18,24,25,26,27,28,29,30,31]. Municipal landfills effluents may besides contain pocket-sized amounts of heavy metals such as: Cd (<0.02–six.five mg/L), Cu (0.005–6 mg/50), Lead (0.01–3.l mg/L), and even Cr6+ (0.04–viii.4 mg/L) [4,31,32,33,34].
Due to the fact that the leachate from municipal landfills comprises a significant amount of organic compounds and shows meaning physicochemical parameters variation depending on their age, they create many technical and technological problems during their handling. For this reason, various physicochemical and biological methods are used to treat the leachate. The landfill leachate could exist treated with: ozone after coagulation treatment [vii], ozonation [21], hydrodynamic cavitation [11], catalytic oxidation (by using Ni/Al2Oiii as the catalyst) by supercritical water oxidation [12], coagulation-flocculation, chemic coagulation and reverse osmosis organisation [13,30], hybrid coagulation-nanofiltration procedure [20], electrocoagulation [15], electrochemical oxidation [17], photoelectrochemical treatment in a continuous period reactor [16], coagulation and Fenton reagent, UV, H2Oii and UV/H2O2 process [35,36,37].
The presented physicochemical methods crave the utilize of physical factors (e.g., pressure, electric current etc.), diverse chemical compounds (coagulants, catalysts, etc.) devices and conditions in order to obtain high purification efficiency. Not all of these methods have found practical application due to, sophisticated technical solutions or complicated cleaning procedures implemented.
The results reported in literature indicate that the treatment of leachate requires biological procedures with activated sludge [38,39] and even phytoremediation methods [31]. Biological methods are characterized by varying effectiveness, which is related to higher biodegradability of leachate from young landfills compared to the old ones (reduction in the BODv/COD ratio where BOD5 is v-day Biochemical Oxygen Need) [twoscore]. Therefore, landfill leachate introduced into biological wastewater treatment plants may have a negative influence on the microorganisms of the activated sludge and reduce the treatment efficiency. In particular, some chemical compounds present in landfill leachate, such as chlorobenzene, dichlorobenzene, chlorophenols, chloroaniline, toluene, ethylbenzene, xylene, phthalates and polycyclic effluvious hydrocarbons (PAHs) [twoscore,41] have a negative influence on the activeness of activated sludge. For this reason, directly application of biological methods for leachate treatment before prior implementation of physicochemical methods is not always possible. Biological methods seem to be the near environmentally friendly, unfortunately, may evidence variable effectiveness due to changing concentrations of pollutants in the treated leachates.
Currently, more and more attention is being paid to such methods of leachate treatment which are environmentally friendly and do not cause additional negative effects. This concept is ideally suited to the method using potassium ferrate (VI) (K2FeO4), which is an eco-friendly powerful oxidant with a dual mechanism of action. On the one hand, it acts in the oxidation of organic and partially inorganic pollutants (simultaneous reduction of Iron6+ to Fethree+), and in the coagulation of pollutants or oxidation products and their adsorption on the flocs of hydrated Atomic number 26(OH)3. Due to the fact that the decomposition products of K2FeOfour are atomic number 26 oxides and oxygen, it is defined every bit a light-green oxidant and can exist a promising alternative to the conventional coagulants [42]. Among others, potassium ferrate (Half dozen) has been engaged for degradation of endocrine-disrupting compounds (EDCs), decomposition of surfactants (SPCs), personal intendance products (PCPs), pharmaceuticals [43], and also for oxidation of cyanides (CN−) originating from the mining and processing of gilt ore, degradation of natural organic matter (NOM), oxidation of North,N–diethyl–three–toluamide (DEET), many dyes (Methylene Blue, Orangish Ii, Vivid Cherry X-3B, Acid Green 16), removal of algae [44] and for wastewater handling [45].
The master objective of the presented study was to assess the possibility of using M2FeO4 for the treatment of leachate from a municipal waste landfill and to select the most favorable conditions (pH, KiiFeOiv conc., reaction time) for the handling of leachate ensuring the maximum reduction of the COD value. Comparative studies were also carried out with the use of conventional coagulants (FeSO4 × 7H2O, FeCl3 × 6H2O) containing an equivalent corporeality of iron (in relation to the amount independent in the near favorable dose of K2FeOiv) and the effect of the iron salts used on the concentration of Total Coli Count (TCC), Most Probable Number of fecal enterococci (MPN) and Total Proteolytic Count (TPC) in the treated leachate.
two. Materials and Methods
2.1. Chemicals
Envifer® (Nano Atomic number 26, Zidlochovice, Czech republic) was engaged every bit the Yard2FeO4 source. Due to its chemical instability the content of M2FeO4 in Envifer® was specified direct before the procedures provided in the Analytical Procedures section. Envifer® was entirely characterized (UV-VIS spectrum, free energy-dispersive X-ray spectroscopy (EDXS) analysis, scanning electron microscopy (SEM) analysis) previously [46]. To adjust the leachate sample pH, 5% and 20% solutions of HtwoSO4 (AvantorTM, Gliwice, Poland) was used. Solid FeSO4 × 7H2O and FeCliii × 6H2O (Chempur, Piekary Śląskie, Poland) were applied as conventional coagulants. For sludge flocculation a 0.05% solution of anionic flocculant Furoflock CW277 (Chemische Fabrik Wocklum Gebr. Hertin GmbH&Co. KG, Balve, Germany) was employed. All chemicals (except K2FeO4) were of analytical grade. Additionally, deionized h2o (<2 µS/cm) was used for preparation and dilution of the solutions.
two.2. Origin and Physicochemical Parameters of the Landfill Leachate
Leachate from the quondam (>10 years) municipal waste landfill located in southern Poland was investigated. The effluents were collected in summer during the rainy season (air temperature 25 ± i °C, precipitation viii mm of water column) from the effluent reservoir, where it flowed through a system of drainage pipes. Fresh leachate (arrival) was collected inside 24 h, every 60 minutes, into sterile i 50 bottles, which were stored at the temperature of four ± 1 °C without fixing the leachate earlier farther investigation. The average sample of the leachate used for the test was obtained by mixing 1 L of unit samples in a sterile 25 L canister. The raw landfill leachate was analyzed as described in the Belittling Procedures department.
two.3. Appliance and Experiment Conditions
All experiments were conducted at a constant temperature (19 ± 1 °C), in beakers containing 250 ± ane mL of tested leachates. The samples were mixed using a magnetic stirrer (MS11, Wigo, Pruszków, Poland) at a constant speed of 250 rpm at the oxidation/coagulation stage and fifty rpm at the flocculation stage. The experiments with MtwoFeO4 were carried out in such a manner that K2FeOiv was added to the measured volume of wastewater, the pH was corrected with 20% H2SO4 and the reaction was carried out for the causeless time span. The quantity of KiiFeO4, pH and reaction time were prepare as predetermined at the stage of planning the experiments. After the oxidation/coagulation procedure was completed, the pH was adjusted to 9.0 ± 0.one in each experiment using 20% NaOH in order to precipitate the Fe3+ ions as Fe(OH)three. Subsequently, 0.25 mL of 0.05% Furoflock CW277 solution (anionic flocculant) was added, and the stirring speed was decreased to 50 rpm. Later on thirty sec, stirring was halted in society to sediment the formed precipitate. A sample of the liquid above the precipitate was nerveless and filtered using a 0.45 µm PTFE syringe filter before COD decision. The filtrate was analysed co-ordinate to the process provided in the Analytical Procedures department. Nether the virtually favorable conditions of reducing the COD value (pH, G2FeO4 conc., reaction time), a verification experiment was carried out using sterile glass and laboratory equipment. In this instance, a sample of treated leachate above the sediment was collected without filtering it through a 0.45 µm PTFE syringe filter and microbiological tests were performed. Under the same weather (pH, reaction time), comparative tests were performed using an equivalent iron dose (GtwoFeO4 vs. FeSO4 × 7H2O and FeCl3 × 6H2O). In each case the treated leachate was analyzed equally described in the Belittling Procedures section.
two.4. Analytical Procedures
Before performing the tests the chromite titration method was engaged to specify the content of KtwoFeO4 in Envifer®. The in a higher place method is composed of oxidizing Cr(OH)4 − ions using FeO4 2− under extremely alkaline conditions, which results in the generation of Iron(OH)3, CrO4 2−, OH−. The G2FeOiv content in technical grade production (%) was calculated using the post-obit formula:
where
and
are the concentration (0.0850 mol/L) and the volume (mL) of the standard Mohr'southward common salt (ammonium fe(2) sulphate, (NHiv)2Fe(Then4)2) solution,
is 198.04 g/mol, and
represents the sample weight (g) [47]. The determination of the K2FeOiv content in Envifer® was also carried out spectrophotometrically (Cary® 50 UV-VIS, Varian Inc., Melbourne, Australia) [48]. In this case, an Envifer® sample (with accuracy ± 0.001 1000) was dissolved in deionized water, and the book was adapted to 100 mL in a volumetric flask. Subsequently, the sample was filtered (0.45 µm) into a quartz cuvette (light path = 10 mm) and the absorbance values at λ = 505 nm was measured instantaneously. The K2FeO4 content in Envifer® (%) was specified according to the following formula:
where A is the absorbance at 505 nm,
is 198.04 grand/mol, 1070 is the tooth absorbance coefficient, G−one cm−1, and
represents the sample weight (one thousand).
The pH-values and temperature were measured using an Inolab® pH/Ion/Cond/Temp 750 chiliad and SenTix® 81 electrodes (WTW, Weilheim in Oberbayern, Germany) [49]. The landfill leachate COD values were evaluated employing a dichromate method and the PF-eleven spectrophotometer [l]. TOC was assayed using the tube test kit Nanocolor® TOC 60, while the end-point was specified using the PF-eleven spectrophotometer. TOC assessment was conducted in two stages. In the offset 1, inorganic carbon was eradicated from the samples by calculation NaHSOiv and stirring the sample (500 rpm, 10 min). In the second stage, organic compounds were degraded by awarding of Na2S2Oeight at 120 °C for 120 min, and thymol blue absorbance variations of sodium table salt solution were measured spectrophotometrically at λ = 585 nm [51]. Determination of Total Nitrogen (TN) was performed by two-stride spectrophotometric method using Nanocolor® Total Nitrogen 220 test tube kit (Macherey-Nagel, Düren, Germany). In the first stage, the wastewater sample was mineralized (Na2StwoO8, H2So4, 120 °C, 30 min), and in the second stage, spectrophotometric decision of nitrogen compounds after their reaction with 2,six–dimethylphenol (DMP, also usually known equally 2,6–xylenol), in a mixture of H2So4 and H3PO4 were carried out [52]. Determination of Total Phosphorus (TP) was performed after effluent sample mineralization (Na2StwoO8, HtwoSofour, 120 °C, thirty min) by using a test tube kit Nanocolor® ortho– and full Phosphate fifteen, with spectrophotometric endpoint detection using a PF-eleven apparatus (Macherey-Nagel, Düren, Germany) [53]. The dilution of leachate samples before microbiological enumeration was performed according to ISO 6887-i:2017 [54]. The enumeration of Full Coli Count (TCC, CFU/mL), Total Proteolytic Count (TPC, CFU/mL) and the Nearly Probable Number of faecal enterococci (MPN/100 mL) were determined co-ordinate to ISO 4832:2006 [55], PN-75/C-04615/17:1975 [56] and PN-C-04615-25:2008 [57], respectively. For the precipitation of gelatin in the Frazier's medium, Frazier'southward reagent (the mixture of HgCl2, HCl and H2O) was used.
two.5. Response Surface Methodology
Central Composite Design (CCD) and Response Surface Methodology (RSM) were engaged to specify the most favorable atmospheric condition for lowering the COD landfill leachate value. The optimization of the COD removal procedure consisted of determining the numerical values of three independent variables (pH, K2FeO4 dose and reaction time) for which the value of the dependent parameter (COD) was the everyman. Based on the literature data on the implementation of Grand2FeOfour for the treatment of wastewater from various sources and taking into account the value of the redox potential for the FeOfour 2− ion (East° = +two.20 V in acidic and East° = +0.72 V in neutral media) and own experience, several preliminary experiments were carried out. The results of these experiments made it possible to judge the pH, K2FeO4 and reaction fourth dimension adopted at the stage of planning experiments with the use of CCD. Therefore, the following values of input parameters were investigated: pH in the range 2–six, M2FeO4 dose 0.2–0.4 one thousand/50 and reaction time x–20 min. The values of the remaining variables, including i.e., temperature, stirring speed, and volume of the treated wastewater sample were set as abiding in each experiment, respectively. Table 1 reports the set-up of the sixteen experiments designated by using CCD.
The obtained empirical findings (the arithmetics hateful of 3 runs was adopted) were investigated statistically; and the bear on of the independent (input) variables (pH, concentration of K2FeOiv (g/Fifty), and reaction fourth dimension (min)) on the dependent (output) parameter (COD, 1000 Otwo/L) was illustrated every bit a response surface graph. For the most favorable values of the iii input parameters, an experimental verification of the model was carried out (additionally, the COD changes later on 25 min, 30 min, 35 min and 40 min reaction time were investigated).
iii. Results and Discussion
iii.one. Physicochemical Parameters of the Landfill Leachate and K2FeOfour
The physicochemical analysis of technical grade potassium ferrate (Six) showed that it independent 40% of pure Chiliad2FeO4. Additionally, previous research revealed that it was composed of 47.31% ± 1.50% Yard, fifteen.00% ± 0.45% Iron, and 37.69% ± 5.20% O, along with impurities such as Yard2O and ferrous compounds other than K2FeOfour (i.east., KthreeFeOiv and KFeO2). Information technology was probably related to the method used at the stage of its synthesis [46].
Table 2 presents chosen physicochemical and microbiological variables of the landfill leachate.
Initiatory specification of the chosen physicochemical and microbiological parameters of the landfill leachate unveiled that they were slightly alkali metal (pH = 8.9) and contained a certain amount of organic compounds expressed as chemical oxygen demand and full organic carbon (COD 770 mg Oii/L and TOC 230 mg/L, respectively). Additionally, the content of organic (and probably inorganic) nitrogen compounds in the tested leachates was specified by the content of full nitrogen and phosphorus (TN 120 mg/L and TP 12 mg/Fifty, respectively).
On the other hand, the conducted microbiological tests showed significant contamination of the investigated leachates with coliforms, fecal bacteria and proteolytic bacteria (TCC half dozen.eight log CFU/mL, MPN iv.0 log/100 mL and 4.iv log CFU/mL, respectively). The obtained test results are comparable with the previous findings, especially for leachate from quondam landfills, for which a decrease in the value of pollution indicators was observed. Generally, in the instance of pH-value values were 4–9.five [4,five,vi,seven,8,9,10,eleven,12], for COD 100–84 300 mg O2/L [4,13,xiv,xv,sixteen] and for TOC 40–13 610 mg/L. In addition, for TN and TP, the values were 350–4.370 mg/L and 1–655 mg/Fifty, respectively [xviii,24,25,26,27,28,29,thirty,31]. The parameter values presented in Table 2 betoken that the tested leachate did come from the old landfill and was collected during the rainy flavour, every bit presented in the section concerning origin and physicochemical parameters of the landfill leachate. Other studies indicated the presence of pathogenic bacteria, not but in the leachate, merely also in groundwater as a outcome of leachate infiltration into the ground (coliform bacteria, Escherichia coli, Enterococci, Pseudomonas aeruginosa). In groundwater, high concentrations of coliform leaner (20,000 CFU/100 mL), Escherichia coli (15,199 CFU/100 mL) and Enterococci (3290 CFU/100 mL) were specified [58]. The conducted studies of leachate revealed that in the consequence of their uncontrolled release, they may have a negative bear upon on the natural environment.
3.ii. CCD/RSM Findings
The employment of CCD and RSM in investigation planning enabled 16 experiments to exist performed (see Table 2). The findings of COD values (g O2/L) linked to each experiment are reported in Table 2 (come across column 5). The lowest COD values (<0.25 chiliad O2/L) were recorded in experiments 9, 12, and xiv (0.245, 0.205, 0.240 g Otwo/50), respectively. In the experiment number 12, the highest dose of K2FeO4 (0.468 yard/Fifty) was used at pH iii.five during 15 min, and the lowest COD value was obtained for the purified effluents (0.205 g O2/L). This indicates a significant influence of the M2FeO4 dose on the COD value of the effluents, along with other parameters (pH-value and reaction time).
Table iii presents the evaluation of the parameters and their influence of the COD of the landfill leachate.
The constant value, pH (L), 10002FeO4 (L) and One thousand2FeO4 (Q), concentration were specified to be statistically valid (p < 0.05), while the pH (Q), Time (L) and Time (Q) were not statistically pregnant (p > 0.05). Moreover, the values of the calculated determination coefficient Rtwo and the adjusted determination coefficient R2 adj (0.8477 vs. 0.7462) depicted the ratio of the variance in the dependent variable (COD) that was foreseen based on the contained variables (pH -value, KiiFeO4 conc. and fourth dimension).
In the example of the existent sewage from the cloth manufacture, R2 and R2 adj reached values of 0.8799 and 0.7999 [45]. In the case of using KtwoFeO4 for the treatment of wastewater from the tanning industry other studies accept reported Rtwo and R2 adj values of 0.77 and 0.59 [59] versus 0.95 and 0.74 in the case of employing Grand2FeO4 for the treatment of constructed sewage containing azo dye Anilan Bluish GRL 250% [60]. A good fit between the empirical and approximated information was observed in the latter study.
Table 4 reports the effect of verifying the adequacy of the model coefficients using ANOVA, which confirmed the statistical significance (p < 0.05) of the main input parameters i.e., pH (L), One thousandiiFeO4 (L) and Chiliad2FeO4 (Q). These findings are also presented graphically in a class of bar chart (come across Figure one).
The estimators of the standardized effects were prioritized according to their accented value; the vertical line pinpoints the minimum absolute value for statistical significance. In the investigated wastewater samples, Yard2FeO4 (L), Thousand2FeOfour (Q), and pH (L), revealed the largest impact on decreasing the COD value under the empirical conditions. The other parameters i.due east., pH (Q), Time (L), fourth dimension (Q) exerted the smallest impact on the COD value. Effigy 2 presents the relationship between the predicted COD value and observed COD value.
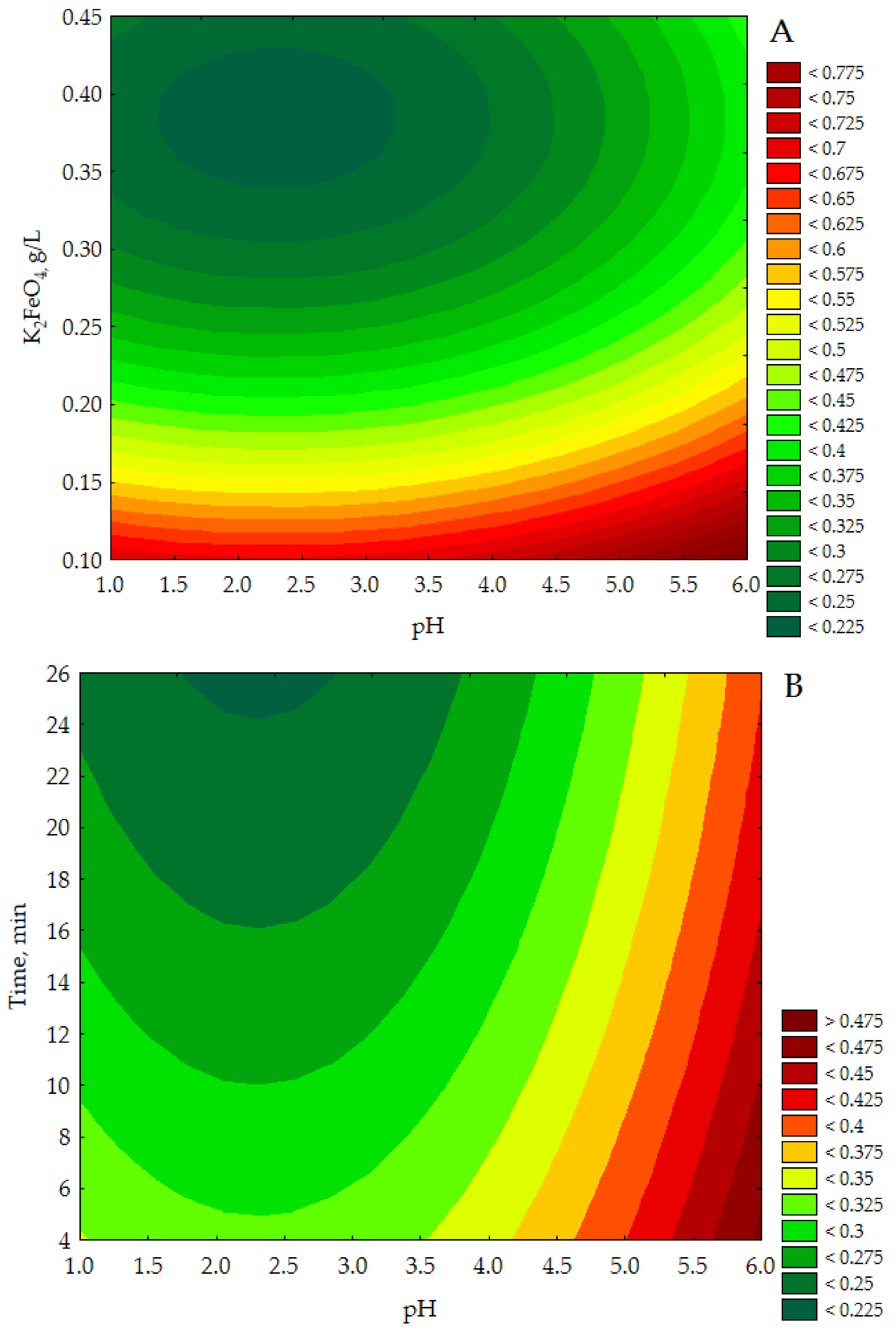
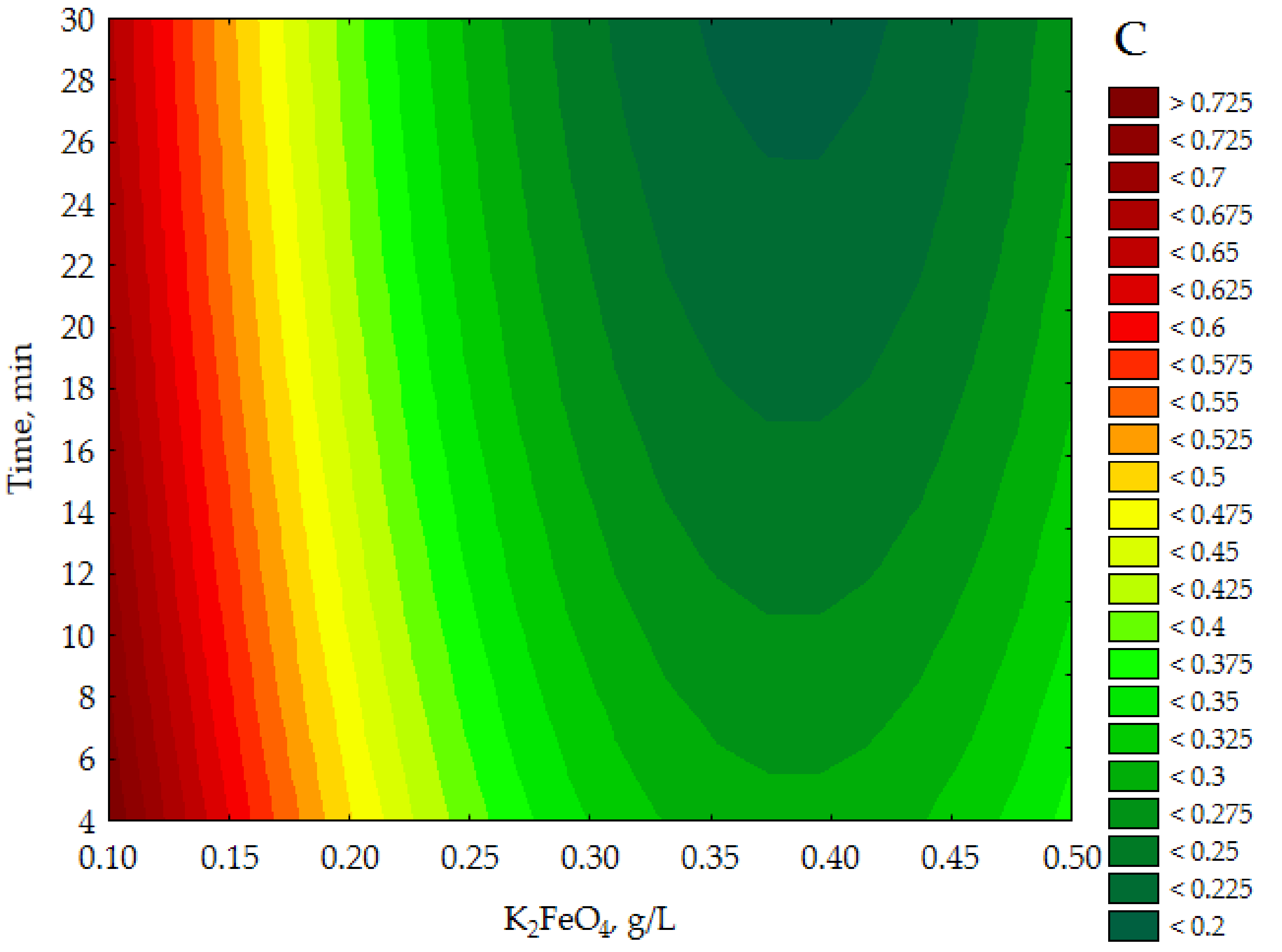
The data presented a linear correlation between the empirical and approximated data in the range of verified COD values. Figure three illustrates the response surface plots for COD with respect to KtwoFeOfour conc. and pH, Time and pH and Time and to K2FeO4 conc. (see Figure 3A–C).
The CCD/RSM study showed (see Effigy 3A) that the lowest COD value (<0.225 g Otwo/L) was obtained for 10002FeOfour approx. 0.34–0.43 g/Fifty and a pH between ane.four–iii.3 with the time parameter set at 15 min. It can be seen in Figure 3B that for a constant dose of One thousand2FeO4 0.300 chiliad/Fifty the lowest COD values (<0.225 g Otwo/50) were specified for pH approx. one.7–2.nine in more than 25 min. In turn, the data presented in Figure 3C indicate that the adoption of a constant pH value of iii.five allows to obtain the everyman COD values of purified effluents (<0.200 chiliad/50) for K2FeO4 conc. approx. 0.35–0.43 g/Fifty over a time greater than 25 min. The presented results of model tests show that the lowest COD values for purified leachates were determined for the highest doses of Thou2FeO4 used in the experiments, in the acidic environment (1.7 < pH < 3.3) for more than 25 min.
The generated examination results stand for to the literature data, which point that the value of the redox potential for the FeO4 2− ion is greater in an acidic surround than in a neutral environment (East° = +ii.20 V in acidic and E° = +0.72 5 in neutral media). More often than not, greater efficiency of the oxidation of organic compounds can be observed, while conducting the oxidation procedure in an acidic surroundings, rather than in a neutral one [43,44]. Some other study indicated that utilise of K2FeOfour for the purification of highly polluted tannery wastewater from leather dyeing processes resulted in the discoloration (98.four% removal), chemic oxygen demand (77.two% removal), total organic carbon (75.vii% removal), and suspended solids (96.nine% removal); the reported values were the smallest when 1.200 g/L KiiFeOiv at pH three within ix min was used [59]. On the other mitt, the awarding of KiiFeOfour for the degradation of trichloroacetic acid and turbidity removal in synthetic water revealed that the highest efficiency achieved for trichloroacetic acid was 24%, while for turbidity the maximum removal efficiency was in the range of 85%–95%. Additionally, the optimum atmospheric condition for initial turbidity, pH, and ferrate (6) dosage were 8.89 NTU, iii, and 4.26 mg/L equally Fe, respectively [61]. Other study indicates that the leachate treatment is besides possible in an element of group i status. In this example the pH value was 10, the dosage of Grand2FeOiv was vi yard/L and the reaction time was 30 min. Unfortunately, the experiment required an boosted use of a stabilizer at a dose iv g/L (sodium silicate, NaiiSiO3). Under those conditions, the COD removal efficiency was only 36% [62] compared to 76.ii% in this written report. An application of Yard2FeOfour in the leachate treatment at the college temperature (thirty °C) past the initial ferrate (VI) to COD mass concentration ratio of 0.fifty, pH 4.00 and reaction fourth dimension 40 min was suggested every bit well. Information technology was stated that the leachate from hazardous waste landfill which was pretreated by K2FeO4 could be directly discharged into the biological handling system. However, COD value of leachates from the refuse incineration plant which was pretreated by K2FeOiv was as much every bit 2861 mg O2/L. These leachates required re-handling before the introduction into the subsequent biochemical handling organization [63].
Moreover, information technology should be taken into account that the total efficiency of removing organic (and partially inorganic) compounds expressed as COD, TOC, TN and TP results not but from their oxidation by Fe+vi, only also to some extent from their adsorption on freshly precipitated Fe(OH)three flocs with a large active surface. In the case of phosphorus compounds (present equally PO4 iii−), it is possible to remove them past co-atmospheric precipitation with Fe3+ ions, which results in the formation of hardly soluble ferric phosphate. To sum up, it should exist stated that nether the experimental conditions, the total efficiency of removing contaminants expressed every bit COD resulted from their oxidation and coagulation and, probably, to some extent from adsorption and co-precipitation. Table v presents the calculated coefficients of the fitted model.
Consequently, the changes in the COD value can exist calculated according to the following formula:
COD (g O2/L) = 1.206468−0.059897(pH) + 0.012988(pH)2 − 4.423640(K2FeOiv) + 5.750741 (K2FeO4)2 − 0.006018(Time) + 0.000073(Time)2
For the about favorable values of the iii input parameters (pH = 2.31, Yard2FeOfour 0.38 g/L and Time 41 min) calculated from the model, the estimated COD value was 162 mg O2/50. In the conducted verification experiment, the COD value was 178 mg O2/L. Bold that the uncertainty of COD determination is ± xv%, the actual COD value of sewage treated under the most favorable conditions is in the range from 151 to 205 mg Oii/L (180 ± 27 mg O2/L), which also includes the estimated value from model for the most favorable pH values, Chiliad2FeO4 and time.
For a constant pH value three.v (see Figure 3B) the lowest COD values (<200 mg O2/L) were obtained after 25 min of reaction time, therefore an additional verification experiment was carried out for the pH value and 10002FeO4 concentration estimated from the model for the most favorable conditions (i.eastward., 2.31 thousand/Fifty and 0.38 g/L, respectively), and the COD was determined after 25 min, xxx min, 35 min and 40 min reaction fourth dimension. The subsequent COD values of the treated effluents were 180 ± 27 mg O2/L, 172 ± 26 mg Oii/L, 170 ± 26 mg O2/50, 168 ± 25 mg O2/L, respectively. Considering the uncertainty of the COD determination (± 15%), it was institute that the COD of the treated leachate was non significantly reduced. Therefore, the most favorable values for the independent parameters i.e., pH = two.3 ± 0.one, K2FeOfour 0.390 ± 0.001 g/Fifty and Time 25 ± 1 min were adopted. Under these conditions, a reduction in TOC, TN and TP was also observed (82.half dozen%, 68.3%, 91.half dozen%, respectively) every bit shown in Table half-dozen (column 3).
3.3. Coagulation/Flocculation Findings and GrandtwoFeOfour Biocidal Properties
Table 6 reports the findings of tests of purified leachates after the awarding of Thousand2FeOiv (under optimal conditions) and FeSOiv × 7H2O and FeCl3 × 6HiiO in an amount equivalent to the dose of iron independent in 0.390 k of KiiFeO4.
The test results revealed that the use of an equivalent dose of fe in the form of FeSOiv × 7HtwoO and FeClthree × 6H2O fabricated information technology possible to reduce the COD, TOC, TN values only past 38.i%, 37.0%, 20.8% (in the example of FeSO4 × 7H2O) and 41.half-dozen%, 45.7%, and 29.2% (in the case of (FeCl3 × 6H2O), compared to K2FeOiv, the application of which was much more than effective (see Table 6, column 3). Additionally, in all cases a reduction of the TP value > 90% was achieved. It is articulate that the removal of impurities from the tested leachates was not only due to coagulation, co-atmospheric precipitation and adsorption (as in the instance with conventional coagulants), but likewise as a result of oxidation procedure using M2FeO4. Additionally, information technology should exist noted that in the example of using conventional coagulants, the efficiency of removing microorganisms was comparable and amounted to 92.1%, 58.2%, 90.0% (in the case FeClthree × 6H2O for TCC, MPN, TPC) and 94.four%, 58.2%, xc.8% (in the case FeSOfour × 7HiiO for TCC, MPN, TPC), respectively. When M2FeO4 was used the microorganism removal efficiency was 99.nine%, 95.eight% and 99.3% for TCC, MPN and TPC, respectively.
The obtained results bespeak an boosted biocidal effect related to the oxidizing backdrop of the FeO4 2− ion in an acidic environs. Other studies revealed that KiiFeOiv can accomplish the disinfection targets (>6 log inactivation of Escherichia coli) at a very low dose (6 mg/L every bit Fe) and over wide working pH range compared to chlorination (10 mg/L as Cltwo) and coagulation (Feii(SOfour)3 iii.iv mg/L as Fe). In wastewater treatment, KtwoFeOiv impale iii log more bacteria in comparison with Al2(SOfour)3 and Fe2(And theniv)three at a similar or even smaller dose [64]. Reports have shown that ferrate (Vi) has excellent disinfectant properties and can inactivate a wide variety of microorganisms at low ferrate (Vi) dosages. Additionally, ferrate (Half-dozen) can disable many chlorine-resistant organisms, such as aerobic spore-formers and sulphite-reducing Clostridia. The ferrate (Half dozen) can deactivate not only Escherichia coli at lower dosages or shorter contact time than hypochlorite, but also Bacillus cereus, Streptococcus bovis, Staphylococcus aureus, Shigella flexneri, Streptococcus faecalis and Salmonella typhimurium, respectively. In plough, ferrate (V) has been proven to exist highly reactive and most 103–ten5 times more reactive to impurities than ferrate (VI), suggesting that the eradication of toxins past ferrate (VI) may be enhanced in the presence of appropriate one-electron-reducing agents. The ferrate (V) has the capability of inactivating biological species and toxins, which cannot exist reached by ferrate (VI) [65]. Since the high reactivity of ferrate (V) allows to inactivate biological species and toxins which cannot be eliminated past ferrate (Vi), information technology seems that this property may likewise exist responsible for inactivation of bacteria.
Moreover, contempo investigations suggested that iron sludge containing iron (Iii) salts and hydroxides that left afterwards the treatment of leachate may be reused for manufacturing of ferrate (Vi) [66]. This possibility of reusing sludge after treatment fits very well to the concept of a circular economy. From the practical and technological betoken of view, it is important to be able to generate ferrate (VI) in situ [67], which reduces the costs of synthesis, transport, storage and handling.
iv. Conclusions
The use of potassium ferrate (6) for the treatment of leachate from a municipal landfill site made it possible to obtain clean leachate characterized by low values of physicochemical (COD, TOC, TN, TP) and microbiological (TCC, MPN, TPC) parameters. Under optimal weather, potassium ferrate (VI) finer decomposed organic compounds present in the leachate and inactivated microorganisms, which was related to its disinfecting consequence. The apply of conventional coagulants in the form of iron (II) and (3) salts allowed for only fractional removal of impurities from the tested leachate. Both in the case of potassium ferrate (VI) and conventional coagulants, iron (2) and (III) hydroxides are formed, which can adsorb impurities or lead to their co-atmospheric precipitation. The maximum efficiency of pollutant removal was obtained with the use of KtwoFeO4 in the process of their oxidation, then coagulation, adsorption and co-precipitation. Moreover, atomic number 26 sludge left after the treatment of leachate may be reused for generation of ferrate. Thus, One thousand2FeO4 can be treated as an effective, multi-functional and environmentally-friendly coagulant for the handling of leachate from municipal landfills.
Author Contributions
Conceptualization, Yard.T.; methodology, M.T., A.B., Grand.B., V.Chiliad.; statistical assay, M.T.; formal analysis, One thousand.T.; data curation, M.T.; writing—original typhoon training, M.T.; writing—review and editing, One thousand.T., Five.K., A.Southward., K.B., A.B.; visualization, M.T.; supervision, K.B. and J.J.; projection administration, Five.K. and A.B.; funding conquering, V.K., A.B. and J.J. All authors take read and agreed to the published version of the manuscript.
Funding
This work was supported by Ministry of Scientific discipline and Higher Educational activity Republic of Poland within statutory funds. This work was besides supported past the Slovak Inquiry and Development Agency (APVV-17-0373, APVV-17-0318) and the Slovak Grant Agency for Science (VEGA 1/0787/xviii).
Acknowledgments
The authors would like to give thanks the anonymous reviewers for their helpful comments.
Conflicts of Involvement
The authors declare no conflict of interest.
References
- Dlugosz, J. Characteristics of the composition and quantity of leachate from municipal landfills—A review. Arch. Waste Manag. Environ. Prot. 2012, 14, 19–30. [Google Scholar]
- Xaypanya, P.; Takemura, J.; Chiemchaisri, C.; Hul, S.; Tanchuling, M.A.N. Characterization of Landfill Leachates and Sediments in Major Cities of Indochina Peninsular Countries—Heavy Metallic Segmentation in Municipal Solid Waste product Leachate. Environs 2018, 5, 65. [Google Scholar] [CrossRef]
- Banel, A.; Zygmunt, B. Volatile fatty acids in a landfill—occurrence and conclusion. Ecol. Chem. Eng. South. 2009, 16, 193–206. [Google Scholar]
- Renou, Due south.; Givaudan, J.; Poulain, South.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Urase, T.; Kikuta, T. Divide estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 2005, 39, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, T.; Bautista, G.L.; Chairez, I.; Córdova, R.I.; Ríos, 50.Due east. Decomposition of toxic pollutants in landfill leachate by ozone later on coagulation treatment. J. Hazard. Mater. 2008, 152, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Şafak, A.; Tünay, O. Bench-scale evaluation of treatment schemes incorporating struvite atmospheric precipitation for young landfill leachate. Waste product Manag. 2008, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- François, V.; Feuillade-Cathalifaud, G.; Skhiri, Northward.; Lagier, T.; Matejka, G. Indicating the parameters of the country of degradation of municipal solid waste. J. Hazard. Mater. 2006, 137, 1008–1015. [Google Scholar] [CrossRef]
- Öman, C.; Junestedt, C. Chemical characterization of landfill leachates—400 parameters and compounds. Waste Manag. 2008, 28, 1876–1891. [Google Scholar] [CrossRef]
- Budi, S.; Suliasih, B.A.; Othman, M.South.; Heng, L.Y.; Surif, S. Toxicity identification evaluation of landfill leachate using fish, prawn and seed establish. Waste Manag. 2016, 55, 231–237. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef]
- Civan, F.; Özaltun, D.H.; Kıpçak, E.; Akgün, One thousand. The treatment of landfill leachate over Ni/Al2O3 past supercritical water oxidation. J. Supercrit. Fluids 2015, 100, 7–fourteen. [Google Scholar] [CrossRef]
- Theepharaksapan, S.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, One thousand. Removal of pollutants and reduction of bio-toxicity in a full scale chemical coagulation and reverse osmosis leachate treatment system. Bioresour. Technol. 2011, 102, 5381–5388. [Google Scholar] [CrossRef]
- Mnif, S.; Zayen, A.; Karray, F.; Bru-Adan, V.; Loukil, S.; Godon, J.J.; Chamkha, M.; Sayadi, S. Microbial population changes in anaerobic membrane bioreactor treating landfill leachate monitored past unmarried-strand conformation polymorphism analysis of 16S rDNA gene fragments. Int. Biodeterior. Biodegrad. 2012, 73, 50–59. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Guo, J.; Wang, Z.; Feng, Q. Landfill leachate treatment using electrocoagulation. Procedia Environ. Sci. 2011, x, 1159–1164. [Google Scholar] [CrossRef]
- Zhao, 10.; Qu, J.; Liu, H.; Wang, C.; Xiao, South.; Liu, R.; Liu, P.; Lan, H.; Hu, C. Photoelectrochemical treatment of landfill leachate in a continuous flow reactor. Bioresour. Technol. 2010, 101, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Englehardt, J.D. Electrochemical oxidation for landfill leachate handling. Waste matter Manag. 2007, 27, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Wang, K.; Zhao, Q.; Wei, L.-L.; Zhang, J.; Yang, J.-C. Characterization of dissolved organic matter during landfill leachate treatment by sequencing batch reactor, aeration corrosive cell-Fenton, and granular activated carbon in series. J. Hazard. Mater. 2010, 179, 1096–1105. [Google Scholar] [CrossRef]
- Ben Yahmed, A.; Saidi, N.; Trabelsi, I.; Murano, F.; Dhaifallah, T.; Bousselmi, L.; Ghrabi, A. Microbial characterization during aerobic biological treatment of landfill leachate (Tunisia). Desalination 2009, 246, 378–388. [Google Scholar] [CrossRef]
- Mariam, T.; Guo, West. Landfill leachate handling using hybrid coagulation-nanofiltration processes. Desalination 2010, 250, 677–681. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Ozonation as polishing treatment of mature landfill leachate. J. Hazard. Mater. 2010, 182, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Ziyang, 50.; Tajuddin, R.Grand.; Farraji, H.; Alifar, N. Co-treatment of landfill leachate and municipal wastewater using the ZELIAC/zeolite constructed wetland system. J. Environ. Manag. 2016, 166, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Niu, C.-One thousand.; Chen, G.; Dong, H.; Liu, Y.; Wan, J.; Chen, A.; Guo, Z.; Yan, M.; Wu, H.; et al. Handling of landfill leachate using immobilized Phanerochaete chrysosporium loaded with nitrogen-doped TiO two nanoparticles. J. Hazard. Mater. 2016, 301, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ziyang, 50.; Zhao, Y.; Tao, Y.; Yu, Southward.; Huili, C.; Nanwen, Z.; Renhua, H. Natural attenuation and characterization of contaminants composition in landfill leachate under dissimilar disposing ages. Sci. Total. Environ. 2009, 407, 3385–3391. [Google Scholar] [CrossRef]
- Oz, N.A.; Yarimtepe, C.C. Ultrasound assisted biogas product from landfill leachate. Waste Manag. 2014, 34, 1165–1170. [Google Scholar] [CrossRef]
- Wu, 50.; Peng, C.; Zhang, South.; Peng, Y. Nitrogen removal via nitrite from municipal landfill leachate. J. Environ. Sci. 2009, 21, 1480–1485. [Google Scholar] [CrossRef]
- Kargi, F.; Catalkaya, East.C. Electrohydrolysis of landfill leachate organics for hydrogen gas production and COD removal. Int. J. Hydrog. Energy 2011, 36, 8252–8260. [Google Scholar] [CrossRef]
- Tsarpali, Five.; Dailianis, Southward. Investigation of landfill leachate toxic potency: An integrated approach with the use of stress indices in tissues of mussels. Aquat. Toxicol. 2012, 58–65. [Google Scholar] [CrossRef]
- Isaka, 1000.; Yoshie, S.; Sumino, T.; Inamori, Y.; Tsuneda, S. Nitrification of landfill leachate using immobilized nitrifying leaner at depression temperatures. Biochem. Eng. J. 2007, 37, 49–55. [Google Scholar] [CrossRef]
- Tatsi, A.; Zouboulis, A.; Matis, K.; Samaras, P. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Jones, D.; Williamson, Grand.; Owen, A. Phytoremediation of landfill leachate. Waste Manag. 2006, 26, 825–837. [Google Scholar] [CrossRef]
- Lema, J.Yard.; Mendez, R.; Blazquez, R. Characteristics of landfill leachates and alternatives for their handling: A review. Water Air Soil Pollut. 1988, xl, 223–250. [Google Scholar]
- Tatsi, A.; Zouboulis, A.I. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv. Environ. Res. 2002, six, 207–219. [Google Scholar] [CrossRef]
- Salem, Z.; Hamouri, Grand.; Djemaa, R.; Allia, K. Evaluation of landfill leachate pollution and treatment. Desalination 2008, 220, 108–114. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Płonka, I.; Barbusiński, Grand.; Amalio-Kosel, M. Comparison of Landfill Leachate Handling Efficiency Using the Advanced Oxidation Processes. Arch. Environ. Prot. 2013, 39, 107–115. [Google Scholar] [CrossRef]
- Barbusiński, 1000.; Pieczykolan, B. COD removal from landfill leachate using Fenton oxidation and coagulation. Archit. Civ. Eng. Environ. 2010, iv, 93–100. [Google Scholar]
- Pieczykolan, Thou.B.B. COD removal from landfill leachate using H2O2, UV radiations and combination these processes. Environ. Prot. Eng. 2012, 38, 5–12. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Barbusiński, Yard.; Płonka, I. Consequence of landfill leachate on the biological treatment of wastewater. Przem. Chem. 2011, ninety, 1555–1559. [Google Scholar]
- Barbusiński, K.; Pieczykolan, B.; Kościelniak, H.; Amalio, M. Effect of landfill leachate on the efficiency of municipal sewage treatment and on the properties of activated sludge. Ochrona Srodowiska 2010, 32, 33–38. [Google Scholar]
- Kulikowska, D. Characterization of organics and methods treatment of leachate from stabilized municipal landfills. Ecol. Chem. Eng. S. 2009, sixteen, 389–402. [Google Scholar]
- Paxéus, N. Organic compounds in municipal landfill leachates. Water Sci. Technol. 2000, 42, 323–333. [Google Scholar] [CrossRef]
- Sharma, V.Yard. Potassium ferrate (Six): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Kliś, Southward.; Barbusiński, M.; Thomas, M.; Mochnacka, A. Application of potassium ferrate (Vi) in the treatment of selected water and wastewater pollutants—Brusque review. Arch. Civ. Eng. Environ. 2020, 13, 129–138. [Google Scholar] [CrossRef]
- Kliś, Southward.; Barbusiński, K.; Thomas, M.; Mochnacka, A. Application of potassium ferrate (VI) for oxidation of selected pollutants in aquatic environment—Short review. Arch. Civ. Eng. Environ. 2019, 12, 129–137. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D. Treatment of Real Textile Wastewater by Using Potassium Ferrate (VI) and Atomic number 26 (III)/H2O2. Application of Aliivibrio Fischeri and Brachionus plicatilis Tests for Toxicity Assessment. Fibres Text. East. Eur. 2019, 27, 78–84. [Google Scholar] [CrossRef]
- Thomas, Yard.; Barbusinski, 1000.; Kliś, Southward.; Szpyrka, Eastward.; Chyc, M. Synthetic Cloth Wastewater Treatment using Potassium Ferrate (VI)—Application of Taguchi Method for Optimisation of Experiment. Fibres Text. East. Eur. 2018, 26, 104–109. [Google Scholar] [CrossRef]
- Schreyer, J.Chiliad.; Thompson, K.West.; Ockerman, L.T. Oxidation of Chromium (Iii) with Potassium Ferrate (Vi). Anal. Chem. 1950, 22, 1426–1427. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Wang, Y.-S.; Liu, C.-H. Preparation of Potassium Ferrate from Spent Steel Pickling Liquid. Metals 2015, 5, 1770. [Google Scholar] [CrossRef]
- ISO. 10523:2008 Water Quality. Determination of pH. Available online: https://www.iso.org/standard/51994.html (accessed on five October 2020).
- ISO. 15705:2002 Water Quality. Determination of the Chemic Oxygen Demand Index (ST-COD). Small-scale-Scale Sealed-Tube Method. Bachelor online: https://www.iso.org/standard/28778.html (accessed on v October 2020).
- Test 985094. TOC sixty. Macherey-Nagel GmbH & Co. KG 5, Düren, German. Available online: http://ftp.mn-net.com/english/Instruction_leaflets/NANOCOLOR/985094en.PDF (accessed on x August 2020).
- Exam 985088. Total Nitrogen TNb 220. Macherey-Nagel GmbH & Co. KG five, Düren, Germany. Available online: https://www.mn-cyberspace.com/media/pdf/1a/bf/a1/Didactics-985088-Tube-test-NANOCOLOR-full-Nitrogen-TNb-220.PDF (accessed on 10 August 2020).
- Test 985080. Ortho- and Total Phosphat fifteen. Macherey-Nagel GmbH & Co. KG v, Düren, Germany. Available online: https://www.mn-cyberspace.com/media/pdf/a8/a4/4c/Educational activity-985080-Tube-examination-NANOCOLOR-ortho-and-total-Phosphate-15.PDF (accessed on x August 2020).
- ISO 6887-ane:2017. Microbiology of the Food Chain-Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination-Role 1: General Rules for the Grooming of the Initial Pause and Decimal Dilutions. Available online: https://world wide web.iso.org/standard/63335.html (accessed on five October 2020).
- ISO 4832:2006. Microbiology of Food and Fauna Feeding Stuffs-Horizontal Method for the Enumeration of Coliforms-Colony-Count Technique. Available online: https://www.iso.org/standard/38282.html (accessed on 5 October 2020).
- PN-75/C-04615/17:1975 Microbiological Tests. Determination of Proteolytic Bacteria by Frazier'south Method; The Polish Committee for Standardization: Warsaw, Poland, 1975.
- PN-C-04615-25:2008 Water and Wastewater. Microbiological Tests. Part 25. Determination of Fecal Enterococci past Tube Exam; The Polish Committee for Standardization: Warsaw, Poland, 2008.
- Grisey, Due east.; Belle, E.; Dat, J.; Mudry, J.; Aleya, L. Survival of pathogenic and indicator organisms in groundwater and landfill leachate through coupling bacterial enumeration with tracer tests. Desalination 2010, 261, 162–168. [Google Scholar] [CrossRef]
- Kozik, V.; Barbusinski, K.; Thomas, Chiliad.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials 2019, 12, 3784. [Google Scholar] [CrossRef]
- Thomas, G.; Barbusinski, K.; Kalemba, K.; Piskorz, P.J.; Kozik, V.; Bak, A. Optimization of the Fenton Oxidation of Constructed Fabric Wastewater using Response Surface Methodology. Fibres Text. Due east. Eur. 2017, 25, 108–113. [Google Scholar] [CrossRef]
- Aslani, H.; Nabizadeh, R.; Nasseri, S.; Mesdaghinia, A.; Alimohammadi, G.; Mahvi, A.H.; Rastkari, Northward.; Nazmara, S. Application of response surface methodology for modeling and optimization of trichloroacetic acid and turbidity removal using potassium ferrate (VI). Desalin. H2o Treat. 2016, 57, 25317–25328. [Google Scholar] [CrossRef]
- Lan, Due south.; Liu, X.; Chen, R.; Wan, Y.; Wu, X.; Zhang, H. Study on pretreatment of landfill leachate by potassium ferrate. Desalin. Water Treat. 2013, 52, 2757–2764. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Qu, Y.; Lin, Y.; Huang, D.; Zheng, Z. Pretreatment of landfill leachate past potassium ferrate (Half dozen). Chin. J. Environ. Eng. 2014, 8, 2451–2455. [Google Scholar]
- Jiang, J.-Q.; Wang, S.; Panagoulopoulos, A. The exploration of potassium ferrate (Half dozen) equally a disinfectant/coagulant in h2o and wastewater handling. Chemosphere 2006, 63, 212–219. [Google Scholar] [CrossRef]
- Sharma, V.Thou. Desinfection operation of Atomic number 26 (Half-dozen) in water and wastewater: A review. Water Sci. Technol. 2007, 55, 225–232. [Google Scholar] [CrossRef]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Cotillas, Southward.; Rodrigo, M.A. Improvements in the Electrochemical Production of Ferrates with Conductive Diamond Anodes Using Goethite equally Raw Cloth and Ultrasound. Ind. Eng. Chem. Res. 2011, l, 7073–7076. [Google Scholar] [CrossRef]
- Ghernaout, D.; Naceur, M.W. Ferrate (Half-dozen): In situ generation and water treatment—A review. Desalin. Water Care for. 2011, xxx, 319–332. [Google Scholar] [CrossRef]
Figure 1. Bar chart of standardized effects (COD, grand O2/L, 3 value, 1 cake, sixteen experiments, MS = 0.0040, L–linear effect, Q–quadratic effect, p–the absolute value of the standardized consequence evaluation).
Figure 1. Bar nautical chart of standardized effects (COD, g Otwo/50, iii value, 1 block, 16 experiments, MS = 0.0040, L–linear result, Q–quadratic effect, p–the absolute value of the standardized consequence evaluation).
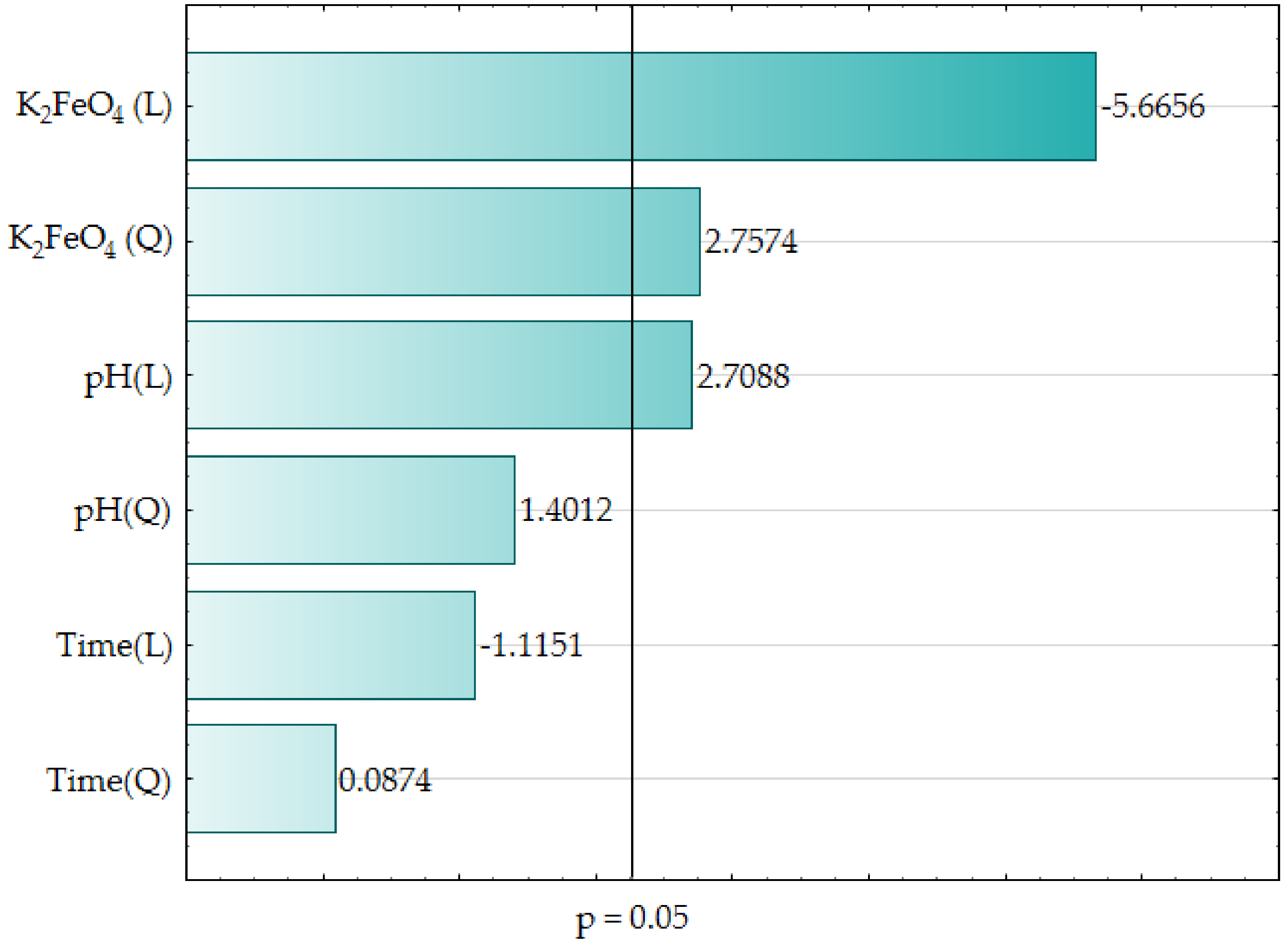
Figure 2. Predicted vs. observed values plots for COD (g O2/L).
Figure 2. Predicted vs. observed values plots for COD (grand Oii/L).
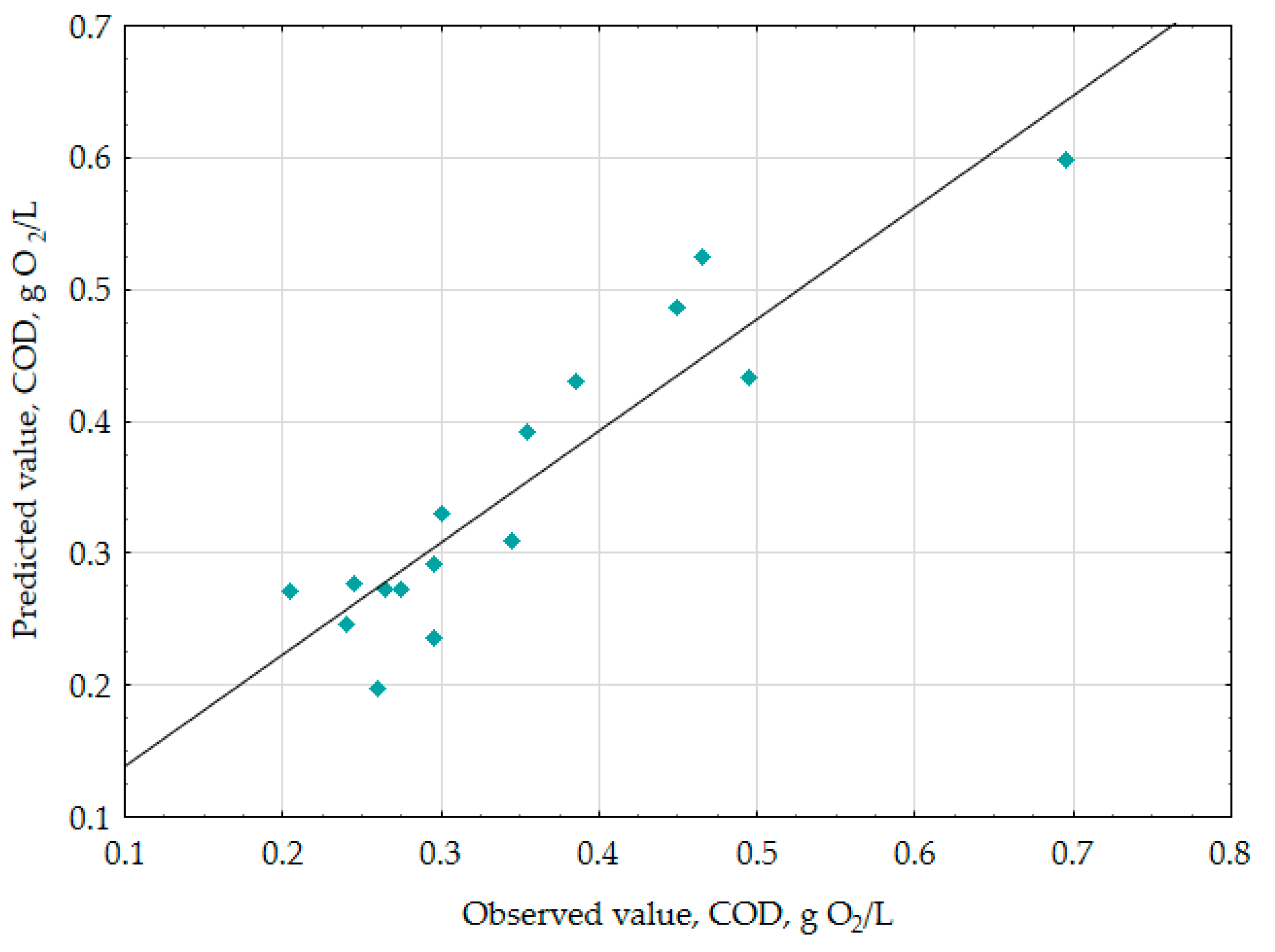
Figure 3. Response surface plots for COD (g Oii/L) with respect to KtwoFeOfour (g/Fifty) and pH (A), fourth dimension (min), and pH (g/L) (B) and fourth dimension (min) and ChiliadiiFeOiv (g/L) (C).
Figure iii. Response surface plots for COD (g O2/L) with respect to Thou2FeOfour (grand/L) and pH (A), time (min), and pH (g/L) (B) and fourth dimension (min) and G2FeOfour (grand/Fifty) (C).
Tabular array 1. Empirical atmospheric condition for the CCD/RSM and result (COD) for landfill leachate (pH 0.98–half dozen.02, Grand2FeO4 0.132–0.468 m/Fifty, Fourth dimension vi.59–23.41 min); (C)-middle of the plan.
Tabular array 1. Empirical weather condition for the CCD/RSM and outcome (COD) for landfill leachate (pH 0.98–6.02, K2FeOfour 0.132–0.468 chiliad/Fifty, Fourth dimension six.59–23.41 min); (C)-center of the plan.
| Run | Experimental Conditions | Experimental Results * | ||
|---|---|---|---|---|
| pH | 1000twoFeOfour (g/L) | Time (min) | COD (g O2/L) | |
| 1 | 2.00 | 0.200 | ten.00 | 0.385 ± 0.058 |
| ii | 2.00 | 0.200 | xx.00 | 0.355 ± 0.053 |
| three | 2.00 | 0.400 | ten.00 | 0.295 ± 0.044 |
| iv | 2.00 | 0.400 | 20.00 | 0.260 ± 0.039 |
| 5 | 5.00 | 0.200 | 10.00 | 0.465 ± 0.070 |
| half dozen | 5.00 | 0.200 | 20.00 | 0.450 ± 0.068 |
| 7 | 5.00 | 0.400 | 10.00 | 0.300 ± 0.045 |
| eight | v.00 | 0.400 | 20.00 | 0.295 ± 0.044 |
| 9 | 0.98 | 0.300 | fifteen.00 | 0.245 ± 0.037 |
| 10 | 6.02 | 0.300 | fifteen.00 | 0.495 ± 0.074 |
| 11 | 3.fifty | 0.132 | 15.00 | 0.695 ± 0.104 |
| 12 | 3.50 | 0.468 | 15.00 | 0.205 ± 0.031 |
| 13 | iii.50 | 0.300 | 6.59 | 0.345 ± 0.052 |
| 14 | 3.50 | 0.300 | 23.41 | 0.240 ± 0.036 |
| 15 (C) | iii.50 | 0.300 | 15.00 | 0.265 ± 0.040 |
| 16 (C) | 3.l | 0.300 | 15.00 | 0.275 ± 0.041 |
Table 2. The specified physicochemical and microbiological parameters of the landfill leachate.
Table two. The specified physicochemical and microbiological parameters of the landfill leachate.
| Parameter | Unit | Upshot * |
|---|---|---|
| pH | – | 8.ix ± 0.ane |
| Chemical Oxygen Demand, COD | mg O2/L | 770 ± 116 |
| Total Organic Carbon, TOC | mg/50 | 230 ± 35 |
| Total Nitrogen, TN | mg/50 | 120 ± 18 |
| Total Phosphorus, TP | mg/Fifty | 12 ± two |
| Full Coli Count, TCC | CFU/mg/Fifty | 6.ii × 106 (6.eight log) |
| Most Probable Number of fecal enterococci, MPNfe | MPN/100 mL | i.1 × 104 (4.0 log) |
| Full Proteolytic Count, TPC | CFU/mL | ii.half-dozen × ten4 (iv.four log) |
Table 3. Statistical parameters of the experiments using CCD/RSM with Statistica 13–evaluation of the furnishings.
Tabular array 3. Statistical parameters of the experiments using CCD/RSM with Statistica 13–evaluation of the effects.
| Parameter | Evaluation of the Effects, COD, 1000 Oii/L, R 2 = 0.8477, R two adj = 0.7462, 3 Parameter, one Block, 16 Experiments, MS = 0.0040 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upshot | Standard Error | p-Value * | −95% Conviction Interval | +95% Conviction Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant Value | 0.2725 | 0.0448 | 0.0002 | 0.1713 | 0.3738 | 0.2725 | 0.0448 | 0.1713 | 0.3738 |
| pH (L) | 0.0931 | 0.0344 | 0.0240 | 0.0153 | 0.1708 | 0.0465 | 0.0172 | 0.0077 | 0.1708 |
| pH (Q) | 0.0584 | 0.0417 | 0.1947 | −0.0359 | 0.1528 | 0.0292 | 0.0209 | −0.0180 | 0.1528 |
| K2FeO4 (Fifty) | 0.1946 | 0.0344 | 0.0003 | −0.2724 | −0.1169 | −0.0973 | 0.0172 | −0.1362 | −0.1169 |
| KiiFeO4 (Q) | 0.1150 | 0.0417 | 0.0222 | 0.0207 | 0.2094 | 0.0575 | 0.0209 | 0.0103 | 0.2094 |
| Time (L) | 0.0383 | 0.0344 | 0.2937 | −0.1160 | 0.0394 | −0.0192 | 0.0172 | −0.0580 | 0.0394 |
| Fourth dimension (Q) | 0.0036 | 0.0417 | 0.9323 | −0.0907 | 0.0980 | 0.0018 | 0.0209 | −0.0454 | 0.0980 |
Table 4. Analysis of the CCD/RSM experiment using Statistica 13—verification of the capability of the model using ANOVA.
Table 4. Analysis of the CCD/RSM experiment using Statistica 13—verification of the adequacy of the model using ANOVA.
| Parameter | Assessment of Effects, COD, g O2/50, R 2 = 0.8477, R 2 adj = 0.7462, 3 Parameter, one Cake, sixteen Experiments, MS = 0.0040 | |||
|---|---|---|---|---|
| SS | MS | F | p * | |
| pH (50) | 0.029567 | 0.029567 | 7.33757 | 0.024045 |
| pH (Q) | 0.007911 | 0.007911 | ane.96337 | 0.194681 |
| KtwoFeOiv (L) | 0.129345 | 0.129345 | 32.09914 | 0.000307 |
| ThousandiiFeO4 (Q) | 0.030637 | 0.030637 | vii.60318 | 0.022207 |
| Time (L) | 0.005011 | 0.005011 | 1.24345 | 0.293696 |
| Time (Q) | 0.000031 | 0.000031 | 0.00764 | 0.932269 |
| Error | 0.036266 | 0.004030 | – | – |
Tabular array v. Coefficients of the fitted model.
Table 5. Coefficients of the fitted model.
| Predictor | Regression Coefficient | Standard Error | t-Value, df = 9 | p-Value | −95% Confidence Interval | +95% Conviction Interval |
|---|---|---|---|---|---|---|
| Intercept | 1.206468 | 0.354637 | 3.401978 | 0.007849 | 0.404223 | 2.008712 |
| pH (L) | −0.059897 | 0.065887 | −0.909076 | 0.387006 | −0.208944 | 0.089151 |
| pH (Q) | 0.012988 | 0.009269 | 1.401202 | 0.194681 | −0.007980 | 0.033957 |
| KtwoFeO4 (Fifty) | −4.423640 | 1.263080 | −3.502263 | 0.006700 | −seven.280926 | −i.566353 |
| M2FeOfour (Q) | v.750741 | 2.085576 | 2.757387 | 0.022207 | 1.032839 | x.468642 |
| Time (50) | −0.006018 | 0.025262 | −0.238234 | 0.817035 | −0.063164 | 0.051128 |
| Time (Q) | 0.000073 | 0.000834 | 0.087398 | 0.932269 | −0.001814 | 0.001960 |
Table 6. Selected physicochemical parameters of treated landfill leachate after GrandiiFeO4, FeSO4 × 7H2O and FeCl3 × 6H2O application.
Table half-dozen. Selected physicochemical parameters of treated landfill leachate after K2FeO4, FeSO4 × 7H2O and FeCl3 × 6H2O application.
| Parameter * | Unit | After ThousandtwoFeOiv Awarding in Optimal Conditions ** | After FeSO4 × 7H2O Application *** | After FeCl × 6H2O Application **** |
|---|---|---|---|---|
| Removal, % (in Brackets) ***** | ||||
| pH | – | ix.0 ± 0.1 | 9.0 ± 0.1 | ix.0 ± 0.i |
| Chemic Oxygen Demand | mg Otwo/L | 180 ± 27 (↓76.2) | 475 ± 71 (↓38.1) | 450 ± 68 (↓41.6) |
| Total Organic Carbon | mg/L | 40 ± vi (↓82.half dozen) | 145 ± 22 (↓37.0) | 125 ± 19 (↓45.7) |
| Total Nitrogen, TN | mg/L | 38 ± half-dozen (↓68.3) | 95 ± 14 (↓20.8) | 85 ± thirteen (↓29.2) |
| Total Phosphorus, TP | mg/L | 1.0 ± 0.2 (↓91.vi) | 0.fifty ± 0.08 (↓95.8) | 0.5 ± 0.08 (↓95.8) |
| Total Coli Count, TCC | CFU/mL | 5.9 × ten2; 2.viii log (↓99.9) | 3.five × 105; v.5 log (↓94.iv) | 4.9 × 10v; 5.7 log (↓92.1) |
| Most Likely Number of fecal enterococci, MPN | MPN/100 mL | 4.6 × ten2; 2.seven log (↓95.8) | 4.6 × 10three; 3.7 log (↓58.2) | iv.6 × 103; 3.seven log (↓58.2) |
| Total Proteolytic Count, TPC | CFU/mL | 1.nine × 102; two.three log (↓99.iii) | 2.iv × 103; 3.4 log (↓90.viii) | ii.6 × ten3; three.iv log (↓90.0) |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 past the authors. Licensee MDPI, Basel, Switzerland. This article is an open access commodity distributed under the terms and atmospheric condition of the Artistic Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/four.0/).
Source: https://www.mdpi.com/1996-1944/13/21/5017/htm
0 Response to "Methodologies for the Analytical Determination of Ferrate(Vi) a Review"
إرسال تعليق